Rapid Point of Care Detection of Tuberculosis
Case ID:
TEC2020-0016
Web Published:
11/8/2022
Executive Summary
Tuberculosis (TB) remains one of the major causes of death from a single infectious agent worldwide, affecting both humans and livestock. Pulmonary TB can be currently diagnosed by various methods including chest radiography, sputum smear microscopy, nucleic acid amplification and cultivation of Mycobacterium tuberculosis (Mtb). However, all these techniques suffer from various issues such as high cost, long diagnosis times or complicated procedures. MSU researchers have recently developed a new technology for detection of TB from clinical samples (sputum, CSF, urine, etc.) that is rapid, inexpensive and can be used as a point-of-care test.
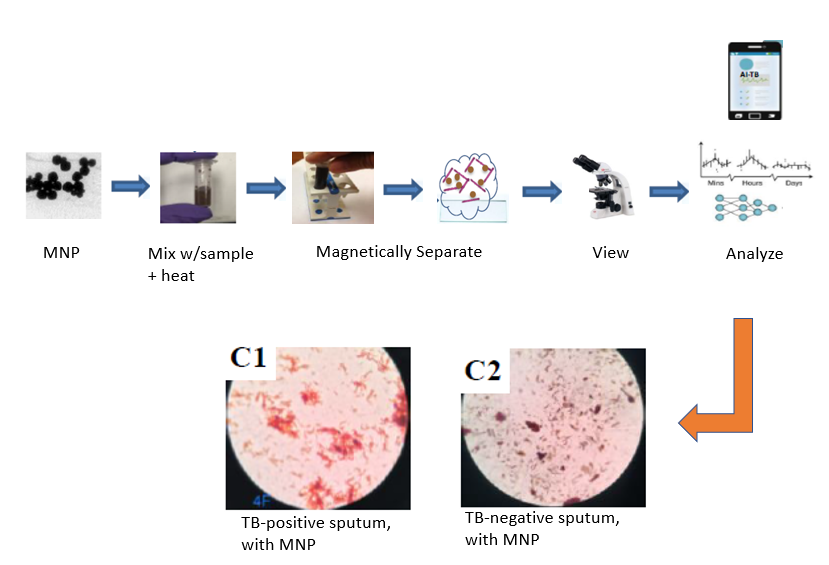
Description of the Technology
This invention involves contacting and heating the unprocessed clinical samples with designed magnetic nanoparticles (MNP) which bind to the TB pathogen (Mtb). The bound Mtb/MNP are separated by a simple magnet and viewed under a microscope. Proprietary software using an AI routine analyzes the images and determines the presence of Mtb with high sensitivity at low detection limits. The results have been compared to the Xpert MTB/RIF assay with excellent agreement.
Benefits
- Easy to use technology
- Rapid detection time (< 1 hr from sample to diagnosis)
- Specificity for TB as high as 85%
- Detection as low as 102 cfu/mL
- Superior results compared to smear technology
Applications
- Rapid point of care TB detection for humans or animals
- Principal can be used for other infectious diseases
Patent Status
Published US application for functionalized magnetic nanoparticles US 2021/0164970 A1
Copyright protection for software and data sets
Licensing Rights
Licensing rights available
Inventors
Dr. Evangelyn Alocilja
TECH ID
TEC2020-0016
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
For Information, Contact:
Jon Debling
Technology Manager
Michigan State University
deblingj@msu.edu