Biosynthetic Route to Phloroglucinol Synthesis
Case ID:
TEC2005-0021
Web Published:
8/10/2023
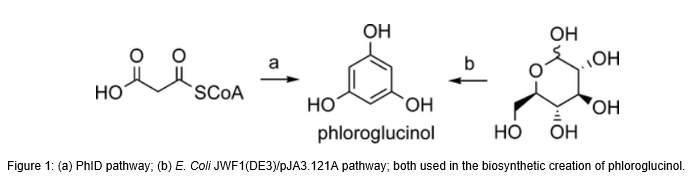
Executive Summary:
Phloroglucinol (1,3,5 trihydroxybenzene) is used in the production of a plethora of applications such as explosive or propellant compounds, medicaments, cosmetics, dyes, and others which make it such a sought-after resource. However, Phloroglucinol is difficult to synthesize by chemical synthesis and alternative routes are desired. Michigan State University researchers have discovered a biosynthetic route to make Phloroglucinol from glucose, while avoiding hazardous chemical and petroleum based starting materials, in addition to doing so in a more efficient and higher yielding manner. that was found to be amenable to microbial synthesis. This method allows for cost-effective production of Phloroglucinol with higher yields and an environmentally clean route.
Description of the Technology:
The invention provides the methods, enzymes, and cells for the biosynthetic production of Phloroglucinol from malonyl-CoA, and ultimately from simple starting materials such as glucose utilizing a recombinant cell comprising phloroglucinol synthase. Specifically, the present invention provides an entirely biosynthetic, anabolic route for Phloroglucinol synthesis that does not require all four of the phlABCD operon enzymes and is capable of commercial Phloroglucinol production using only a phlD enzyme or other Phloroglucinol synthase. In addition to the pathway itself, methods for preparing derivatives of biosynthetic Phloroglucinol were also established, such as that of Resorcinol.
Benefits:
- Efficient biosynthetic route of creating Phloroglucinol
- Sustainable and environmentally clean manufacturing route to important chemicals in large quantities
- Potential for the creation of a domestic supply of Phloroglucinol
- Can be converted to other molecules (e.g. resorcinol) by catalytic hydrogenation
Applications:
- Phloroglucinol can be used for explosive or propellant compounds and compositions
- Phloroglucinol also functions as an antioxidant, stabilizer, and corrosion resistance agent, and is utilized as a coupling agent for photosensitive duplicating paper, as a substitute for silver iodide in rain-making, as a bone sample decalcifying agent, and as a floral preservative.
- Resorcinol can be used for non-explosive or propellant compounds: medicaments, cosmetics, dyes, polymer resin, rubber, adhesive, sealant, coating, composition material, and laminated or bonded materials.
- Resorcinol-aldehyde resin adhesives are especially useful in applications requiring high bond strength, including, e.g.: wooden trusses, joists, barrels, and boats; and aircraft.
- Modified resorcinol-aldehyde resin adhesives are also used as biological wound sealant compositions both on topical wounds and on internal wounds or surgical cuts, e.g., vascular incisions.
Patent Status:
Issued patent US 7,943,362
Licensing Rights:
Full licensing rights available
References:
Journal of the American Chemical Society, 2005
Inventors:
Dr. John W. Frost
Tech Identification:
TEC2005-0021
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
For Information, Contact:
Kenneth Foster
Technology Manager
Michigan State University
foste462@msu.edu